Strand A: Scientific Discovery Programme
Research Programme 2
Identification of therapeutic targets tackling a core cause of synucleinopathies underlying Parkinson-Plus and Parkinson's disease
Lead: Professor David Rubinsztein
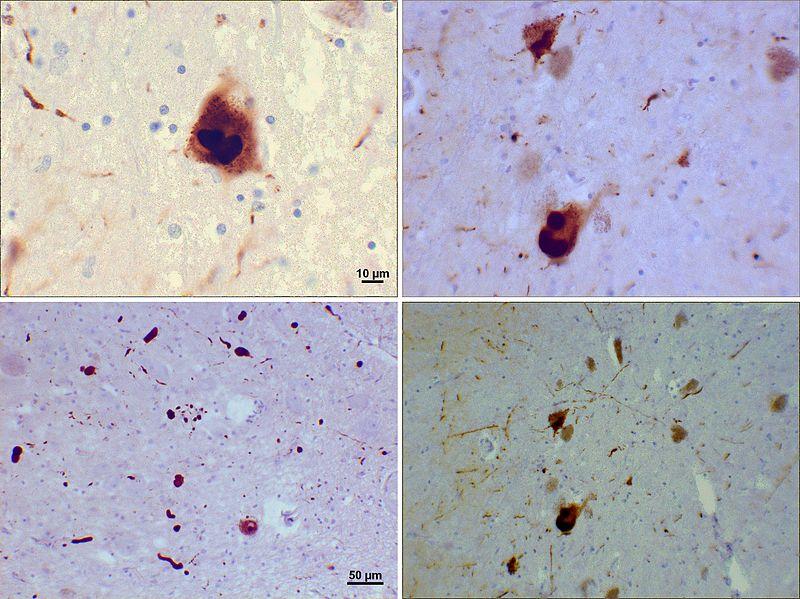
Many forms of Parkinson’s disease (PD) and Parkinson-Plus syndromes, like Lewy Body dementia, are characterized pathologically by the cytoplasmic accumulation of proteinaceous material within aggregates, called Lewy bodies.
One of the major constituents of Lewy bodies is the protein α-synuclein. This protein is likely to be a toxic mediator of pathology in PD and Parkinson-Plus synucleinopathies. For example, α-synuclein gene duplications increase its expression and cause rare cases of autosomal dominant PD. Thus, reducing α-synuclein levels is a potential strategy for synucleinopathies.
The Rubinsztein Laboratory has shown that wild type α-synuclein degradation is regulated by both autophagy and the ubiquitin-proteasome pathway. Importantly, they were the first to show that autophagy-upregulating compounds speed up α-synuclein degradation. Interestingly, they also found that α-synuclein excess impairs autophagy, while a reduction in α-synuclein levels activates this pathway.
The team has also recently described how other PD-associated variants impact protein clearance via autophagy. There is a poor understanding of the druggable targets that modulate α-synuclein levels. This project aims to identify such modifiable pathways. The knowledge of a specific target and pathway can serve as a potent starting point for drug discovery efforts for new treatments against these disorders.
Related publications
- Ashkenazi, A., Bento, C. F., Ricketts, T., Vicinanza, M., Siddiqi, F., Pavel, M., ... & Rubinsztein, D. C. (2017). Polyglutamine tracts regulate beclin 1-dependent autophagy. Nature, 545(7652), 108.
- Bento, C. F., Ashkenazi, A., Jimenez-Sanchez, M., & Rubinsztein, D. C. (2016). The Parkinson's disease-associated genes ATP13A2 and SYT11 regulate autophagy via a common pathway. Nat Commun, 7, 11803. doi:10.1038/ncomms11803
- Ejlerskov, P., Hultberg, J. G., Wang, J., Carlsson, R., Ambjorn, M., Kuss, M., Liu, Y., Porcu, G., Kolkova, K., Friis Rundsten, C., Ruscher, K., Pakkenberg, B., Goldmann, T., Loreth, D., Prinz, M., Rubinsztein, D. C., & Issazadeh-Navikas, S. (2015). Lack of Neuronal IFN-beta-IFNAR Causes Lewy Body- and Parkinson's Disease-like Dementia. Cell, 163(2), 324-339. doi:10.1016/j.cell.2015.08.069
- Frake, R. A., Ricketts, T., Menzies, F. M., & Rubinsztein, D. C. (2015). Autophagy and neurodegeneration. J Clin Invest, 125(1), 65-74. doi:10.1172/JCI73944
- Galluzzi, L., Baehrecke, E. H., Ballabio, A., Boya, P., Bravo-San Pedro, J. M., Cecconi, F., Choi, A. M., Chu, C. T., Codogno, P., Colombo, M. I., Cuervo, A. M., Debnath, J., Deretic, V., Dikic, I., Eskelinen, E. L., Fimia, G. M., Fulda, S., Gewirtz, D. A., Green, D. R., Hansen, M., Harper, J. W., Jaattela, M., Johansen, T., Juhasz, G., Kimmelman, A. C., Kraft, C., Ktistakis, N. T., Kumar, S., Levine, B., Lopez-Otin, C., Madeo, F., Martens, S., Martinez, J., Melendez, A., Mizushima, N., Munz, C., Murphy, L. O., Penninger, J. M., Piacentini, M., Reggiori, F., Rubinsztein, D. C., Ryan, K. M., Santambrogio, L., Scorrano, L., Simon, A. K., Simon, H. U., Simonsen, A., Tavernarakis, N., Tooze, S. A., Yoshimori, T., Yuan, J., Yue, Z., Zhong, Q., & Kroemer, G. (2017). Molecular definitions of autophagy and related processes. EMBO J, 36(13), 1811-1836. doi:10.15252/embj.201796697
- Lopez, A., Lee, S. E., Wojta, K., Ramos, E. M., Klein, E., Chen, J., Boxer, A. L., Gorno-Tempini, M. L., Geschwind, D. H., Schlotawa, L., Ogryzko, N. V., Bigio, E. H., Rogalski, E., Weintraub, S., Mesulam, M. M., Tauopathy Genetics, C., Fleming, A., Coppola, G., Miller, B. L., & Rubinsztein, D. C. (2017). A152T tau allele causes neurodegeneration that can be ameliorated in a zebrafish model by autophagy induction. Brain, 140(4), 1128-1146. doi:10.1093/brain/awx005
- Menzies, F. M., Fleming, A., Caricasole, A., Bento, C. F., Andrews, S. P., Ashkenazi, A., Fullgrabe, J., Jackson, A., Jimenez Sanchez, M., Karabiyik, C., Licitra, F., Lopez Ramirez, A., Pavel, M., Puri, C., Renna, M., Ricketts, T., Schlotawa, L., Vicinanza, M., Won, H., Zhu, Y., Skidmore, J., & Rubinsztein, D. C. (2017). Autophagy and Neurodegeneration: Pathogenic Mechanisms and Therapeutic Opportunities. Neuron, 93(5), 1015-1034. doi:10.1016/j.neuron.2017.01.022
- Rubinsztein, D. C. (2016). Tau toxicity feeds forward in frontotemporal dementia. Nat Med, 22(1), 24-25. doi:10.1038/nm.4029
- Rubinsztein, D. C. (2017). RIPK1 promotes inflammation and beta-amyloid accumulation in Alzheimer's disease. Proc Natl Acad Sci U S A, 114(41), 10813-10814. doi:10.1073/pnas.1715241114
- Zavodszky, E., Seaman, M. N., Moreau, K., Jimenez-Sanchez, M., Breusegem, S. Y., Harbour, M. E., & Rubinsztein, D. C. (2014). Mutation in VPS35 associated with Parkinson's disease impairs WASH complex association and inhibits autophagy. Nat Commun, 5, 3828. doi:10.1038/ncomms4828

