Strand B: Clinical Translational Research
Clinical Programme 1
Understanding mechanisms and heterogeneity of human Parkinson-Plus syndromes.
Lead/Co-lead: Professor James B. Rowe/Professor John T. O'Brien
Progress in therapies against Parkinson-Plus syndromes is held back for three reasons.
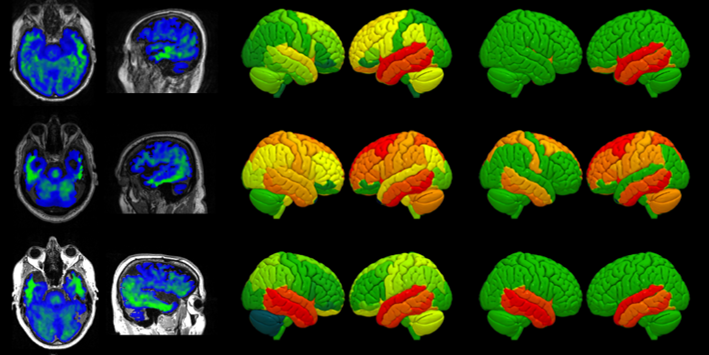
The first is clinical heterogeneity. While classical presentations of PSP, CBS/CBD, MSA and DLB are easy to recognise, these diseases vary widely in survival and complications, and overlap in key features. New treatments require accurate targeting and stratification to reach all possible beneficiaries.
The second problem is the lack of sensitive quantitative biological tools to measure the early impact of Parkinson-Plus pathologies on human brain health and function. These tools are necessary to underpin experimental medicine trials, but are also vital to link preclinical discovery science to the Parkinson-Plus clinical disorders.
The third problem is that Parkinson-Plus is overwhelmingly sporadic (not inherited), which has led to major gaps in knowledge about the early stages of disease, when disease-modifying treatments would have greatest benefit.
This programme meets the urgent need for better quantitative biologic tools and biomarkers to overcome these three obstacles and produce novel and generalisable treatments of Parkinson-Plus syndromes.
Methods
- Pathological differentiation of Parkinson-Plus syndromes
- PET imaging with novel tracers to develop new biomarkers for different syndromes and disease stages
- Blood and cerebrospinal fluid (CSF) markers
- Ultra-high-field magnetic resonance imaging (MRI, 7 tesla)
- Magnetoencephalography (MEG) imaging
- Informatics
Related publications:
- Bevan-Jones, W. R., Cope, T. E., Jones, P. S., Passamonti, L., Hong, Y. T., Fryer, T. D., Arnold, R., Allinson, K. S. J., Coles, J. P., Aigbirhio, F. I., Patterson, K., O'Brien, J. T., & Rowe, J. B. (2017a). [(18)F]AV-1451 binding in vivo mirrors the expected distribution of TDP-43 pathology in the semantic variant of primary progressive aphasia. J Neurol Neurosurg Psychiatry. doi:10.1136/jnnp-2017-316402
- Bevan-Jones, W. R., Surendranathan, A., Passamonti, L., Vazquez Rodriguez, P., Arnold, R., Mak, E., Su, L., Coles, J. P., Fryer, T. D., Hong, Y. T., Williams, G., Aigbirhio, F., Rowe, J. B., & O'Brien, J. T. (2017b). Neuroimaging of Inflammation in Memory and Related Other Disorders (NIMROD) study protocol: a deep phenotyping cohort study of the role of brain inflammation in dementia, depression and other neurological illnesses. BMJ Open, 7(1), e013187. doi:10.1136/bmjopen-2016-013187
- Bevan Jones, W. R., Cope, T. E., Passamonti, L., Fryer, T. D., Hong, Y. T., Aigbirhio, F., Kril, J. J., Forrest, S. L., Allinson, K., Coles, J. P., Simon Jones, P., Spillantini, M. G., Hodges, J. R., O'Brien, J. T., & Rowe, J. B. (2016). [(18)F]AV-1451 PET in behavioral variant frontotemporal dementia due to MAPT mutation. Ann Clin Transl Neurol, 3(12), 940-947. doi:10.1002/acn3.366
- Cope, T. E., Rittman, T., Borchert, R. J., Jones, P. S., Vatansever, D., Allinson, K., Passamonti, L., Vazquez Rodriguez, P., Bevan-Jones, W. R., O'Brien, J. T., & Rowe, J. B. (2018). Tau burden and the functional connectome in Alzheimer's disease and progressive supranuclear palsy. Brain, 141(2), 550-567. doi:10.1093/brain/awx347
- Hall, B., Mak, E., Cervenka, S., Aigbirhio, F. I., Rowe, J. B., & O'Brien, J. T. (2017). In vivo tau PET imaging in dementia: Pathophysiology, radiotracer quantification, and a systematic review of clinical findings. Ageing Res Rev, 36, 50-63. doi:10.1016/j.arr.2017.03.002
- Koychev, I., Gunn, R. N., Firouzian, A., Lawson, J., Zamboni, G., Ridha, B., Sahakian, B. J., Rowe, J. B., Thomas, A., Rochester, L., Ffytche, D., Howard, R., Zetterberg, H., MacKay, C., Lovestone, S., Deep, & Frequent Phenotyping study, t. (2017). PET Tau and Amyloid-beta Burden in Mild Alzheimer's Disease: Divergent Relationship with Age, Cognition, and Cerebrospinal Fluid Biomarkers. J Alzheimers Dis, 60(1), 283-293. doi:10.3233/JAD-170129
- Mak, E., Gabel, S., Su, L., Williams, G. B., Arnold, R., Passamonti, L., Vazquez Rodriguez, P., Surendranathan, A., Bevan-Jones, W. R., Rowe, J. B., & O'Brien, J. T. (2017a). Multi-modal MRI investigation of volumetric and microstructural changes in the hippocampus and its subfields in mild cognitive impairment, Alzheimer's disease, and dementia with Lewy bodies. Int Psychogeriatr, 29(4), 545-555. doi:10.1017/S1041610216002143
- Mak, E., Su, L., Williams, G. B., Firbank, M. J., Lawson, R. A., Yarnall, A. J., Duncan, G. W., Mollenhauer, B., Owen, A. M., Khoo, T. K., Brooks, D. J., Rowe, J. B., Barker, R. A., Burn, D. J., & O'Brien, J. T. (2017b). Longitudinal whole-brain atrophy and ventricular enlargement in nondemented Parkinson's disease. Neurobiol Aging, 55, 78-90. doi:10.1016/j.neurobiolaging.2017.03.012
- Murley, A. G., & Rowe, J. B. (2018). Neurotransmitter deficits from frontotemporal lobar degeneration. Brain. doi:10.1093/brain/awx327
- Passamonti, L., Vazquez Rodriguez, P., Hong, Y. T., Allinson, K. S., Williamson, D., Borchert, R. J., Sami, S., Cope, T. E., Bevan-Jones, W. R., Jones, P. S., Arnold, R., Surendranathan, A., Mak, E., Su, L., Fryer, T. D., Aigbirhio, F. I., O'Brien, J. T., & Rowe, J. B. (2017). 18F-AV-1451 positron emission tomography in Alzheimer's disease and progressive supranuclear palsy. Brain, 140(3), 781-791. doi:10.1093/brain/aww340
- Whitwell, J. L., Hoglinger, G. U., Antonini, A., Bordelon, Y., Boxer, A. L., Colosimo, C., van Eimeren, T., Golbe, L. I., Kassubek, J., Kurz, C., Litvan, I., Pantelyat, A., Rabinovici, G., Respondek, G., Rominger, A., Rowe, J. B., Stamelou, M., Josephs, K. A., & Movement Disorder Society-endorsed, P. S. P. S. G. (2017). Radiological biomarkers for diagnosis in PSP: Where are we and where do we need to be? Mov Disord, 32(7), 955-971. doi:10.1002/mds.27038

